Aeras Bio has a Cell Processing Center for harvesting, culturing, and processing dental pulp stem cells, and is engaged in contract-manufacturing of dental pulp stem cells (specified cell products) obtained from unneeded teeth.
The Cell Processing Center (CPC) is a licensed facility for the manufacture of specified cell products based on the Act on the Securing the Safety of Regenerative Medicine. (Facility number: FA5190002).
A high level of cleanliness is required inside the facility to carry out cell production. For this reason, the environmental control inside the facility includes not only temperature but also room pressure control.
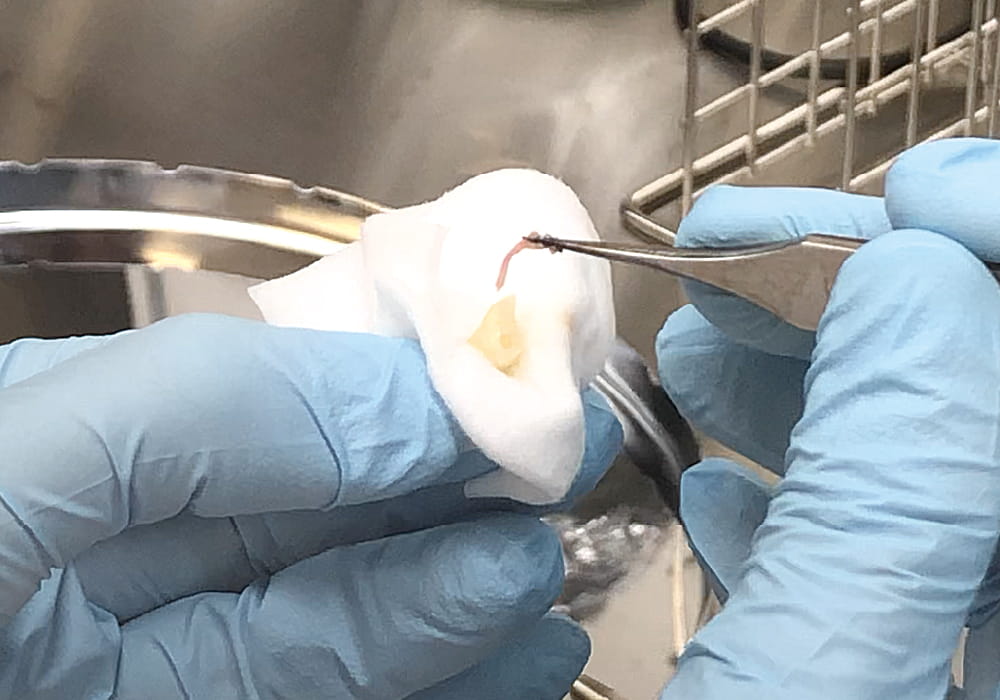
When dental pulp stem cells are collected, they are manipulated inside a piece of equipment called an isolator to prevent contamination of cells by foreign matter and bacteria from the outside to the maximum possible extent during production.
We are also conducting further research and development on processing and preservation technologies for dental pulp stem cells required for pulp regenerative treatment.

Contract manufacturing process flow
(for pulp regenerative treatment)
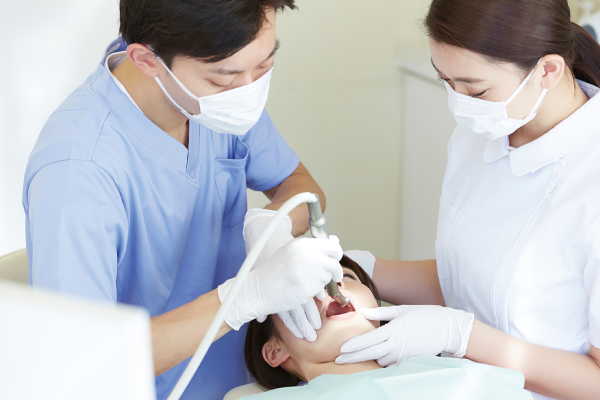
1) Tooth extraction and transportation
Teeth extracted at a dental clinic are sent to our CPC
2) Collection and culture of dental pulp stem cells
Pulp stem cells are harvest from the tooth and expanded to the number required for treatment. (The cultivation period depends on the required number and speed of growth rate, but usually lasts around 1–2 months.)

3) Quality assessment / safety assessment
Before shipping, we evaluate the quality and safety of the cells and confirm that there are no problems before sending the stem cells to the dental clinic.

4) Stem cell transplantation
The stem cells are used in treatment at the dental clinic.
We also accept consultations regarding research and contract-manufacturing using dental pulp stem cells.
If you are interested, please contact us.
